By Greg McConiga

What, exactly, is infrared? According to The American Heritage Dictionary, it’s “electro-magnetic radiation having wavelengths greater than those of visible light and shorter than those of microwaves.†That tells us two things: You can’t see it, and it can carry energy. Okay, fine. So what is a non-contact infrared thermometer, and why can’t I live without it? It’s a great tool with a whole lot of applications around the shop. Some are more practical than others, but we’ll look at quite a few and let you decide what might actually help you in your demanding career. You might just avoid some troubleshooting mistakes, to say nothing of wowing your friends. Speaking of which, if you let them play with it, make sure you warn them that the laser should never be aimed at or near anybody’s eyes as it’s powerful enough to cause retinal damage.
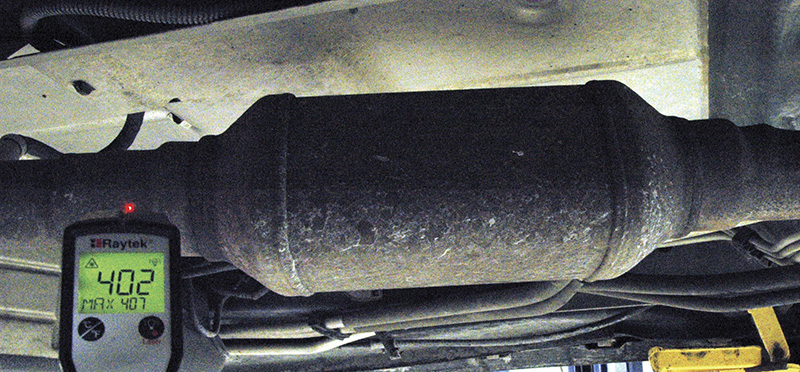
We work on machines with hundreds of systems that require the movement and management of heat. Heat may be intentionally created and managed (for example, the combustion process, turbochargers, exhaust or HVAC systems), or it might be the result of things in the process of destruction (bearings, high-resistance electrical connections, tires or brakes come to mind). Not all heat is equal, as we’ll see, and a standard contact thermometer isn’t always the handiest tool to use, thus the need for an infrared thermometer.
How’s it Work?
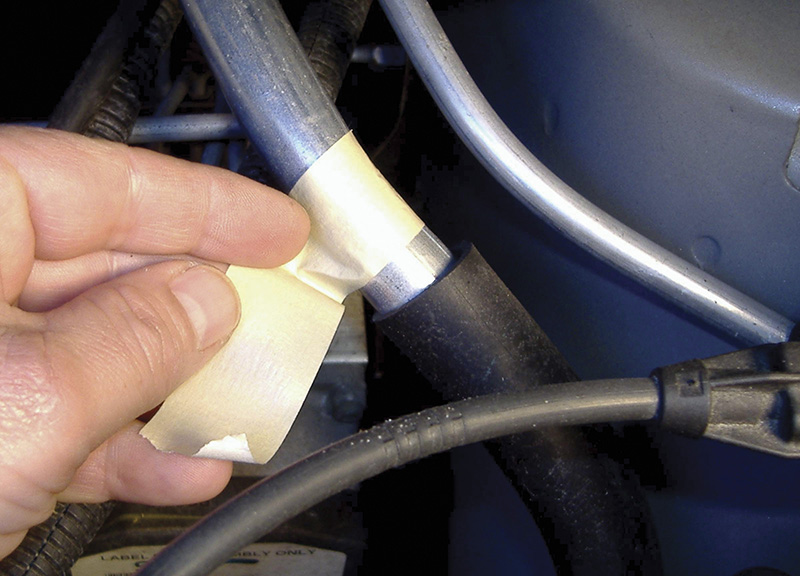
A simple trick that assures a more accurate reading on a shiny surface is the application of ordinary masking tape.
These measuring devices comprise a lens to focus the infrared (IR) energy onto a detector, and supporting circuitry to convert that energy into an electrical signal that can be modified to display as temperature (after compensation for ambient). Two things are in play: the actual temperature of the item you want to measure, and its emissivity. That is, a ratio of the energy radiated by the object under test at a known temperature to the energy emitted by a perfect radiator (or “blackbodyâ€) at the same temperature.
You know how motorcycle racers paint their jugs and heads flat black? Or, how most heat exchangers are the same color? That’s because, as the manual for one IR thermometer puts it, “The amount of infrared energy radiated by an object depends on its emissivity and temperature. The emissivity depends on the material and its surface characteristics.â€
The emissivity of a blackbody is exactly 1.0, with all other objects falling somewhere between 0.0 and 1.0. Most infrared thermometers have the ability to compensate to some degree for the different emissivity values of various materials (see sidebar), but still the higher the emissivity of an object, the more accurate the infrared temperature measurement. It can be quite difficult to find out exactly how hot or cold objects with very low emissivities (below 0.2) are. Polished, shiny metallic surfaces, such as aluminum, are so reflective in the infrared band that accurate temperature measurements aren’t always possible.
Are there ways to trick the IR thermometer? Sure! At temperatures up to about 500 degrees F., a piece of masking tape, with an emissivity of 0.95, can be applied to the surface before the measurement is taken. For higher temperature applications, dull black paint will do the job because its emissivity is pretty close to 1.0. I experimented with Krylon dulling spray (didn’t work), a black magic marker (didn’t work), masking tape (worked pretty well) and flat black paint (worked quite well) for some of the measurements I took. Since customers might not take kindly to having parts of their cars repainted, standard tan-colored masking tape seems to be the most practical solution.
Thermodynamics Stated Simply
About a million years ago, the U.S. Navy kidnapped me and forced me through its nuclear engineering program wherein I was held me down and taught all sorts of things about thermodynamics I really didn’t want to know. While we don’t need to be engineers, we do need to be conversant with some of the characteristics of heat and heat movement. Some basics:
- Heat is a measurement of atomic or molecular energy expressed as a temperature. The faster the molecules of a material are vibrating, the higher the temperature of the material. It can be transmitted by conduction (collision between objects), convection (circulation), or radiation (waves, rays or particles), depending on the materials involved.
- Heat always moves from higher temperatures to lower temperatures in the universal quest for equilibrium.
- Specific heat capacity, also known as specific heat, is the measure of the heat energy required to raise the temperature of a substance by one degree. The amount of energy can be defined for a given mass, a given volume or a given number of molecules(or moles) of a substance. For water, the mass specific heat capacity is about 4,184 joules/degree Kelvin/kilogram, or one Btu per degree per pound in the English customary system. In other words it takes 4,184 joules of energy to raise the temperature of one kilogram of water one degree Kelvin. Specific heat is a physical property of the material itself and doesn’t vary with shape or size; it only varies with the microscopic and chemical structure of the material. For dry air, it’s .24/Btu/lb/degree F. in the customary system
- Sensible heat is heat that you can feel or “sense.†For example, you can feel and measure the change in temperature of the air at the air A/C ducts.
Latent heat, on the other hand, is heat that you cannot feel or sense. Matter exists in one of three “states,†solid, liquid or vapor. Latent heat is gained or lost anytime there’s a change of state. If you want to convert one pound of water to steam, you have to add 970 Btus to the water after it reaches 212 deg. F., which is called the “latent heat of vaporization.†Conversely, if you condense one pound of water from steam, the latent heat of condensation (still 970 Btu per pound) is given off as condensation occurs, but the physical temperature of the steam and water mixture will remain at 212 deg. F. throughout the entire process as the water reforms.
Is that a lot of energy? We know a Btu is defined as the amount of heat needed to raise one pound of water one degree Fahrenheit, so, yes, 970 Btus is quite a bit of energy!
Water or water/coolant mixes and refrigerants all have latent heats of vaporization, condensation and fusion. We don’t really care about the latent heat of the engine coolant since that liquid doesn’t change state unless something has gone badly awry. Since we don’t work with icemakers, we don’t care about the latent heat of fusion. The latent heat of vaporization and condensation, however, is what makes it possible for A/C systems to move heat out of the cabin of a vehicle.
Why does a liquid absorb extra energy in the form of latent heat to vaporize and why does it give off that energy when it condenses? Using water as our example, it takes extra energy to give a water molecule enough moxie to break free of the attraction of its neighbors and achieve the “escape velocity†necessary to knock its way past the molecules lying just above the surface of the boiling liquid. When the steam condenses, it has to lose that energy in order to reform into liquid.
Refrigerants use this property to move heat. The latent heat of vaporization for R-12 is about 72 Btu per pound (166.95 kJ per kg), and for R-134a it’s about 93 Btu per pound (215.9 kJ per kg.)
So What?
Here’s why we should care about these principles:
- Heating different things with the same amount of energy doesn’t mean that the temperature of those things will rise the same amount. In part, different materials react differently because of specific heat.
- The amount of temperature rise or drop you see on different sides of a heat exchange system isn’t and shouldn’t necessarily be equal. You’ll see the air temperatures in and out of the condenser rise more than the temperature drop of the liquid side of the core would indicate because of the properties of sensible heat (that which you can easily measure), latent heat (of condensation), and specific heat (of the refrigerant and the air). What you look for are trends, and you’ll have to experiment with several properly operating cars to establish your own set of baselines that’ll help tip you off to trouble.
- When you’re testing air-to-liquid heat exchangers, you need to be aware of what happens to temperature when you change flow rates and volumes. A partially restricted liquid side will result in a very large temperature drop on the liquid side, inlet to outlet. On the air side, the extra temperature rise will be very small. Too much “dwell†time in the core overcools the liquid and under-heats the air side. If the air side is restricted, the liquid side inlet-to-outlet temperatures won’t vary much, but the air side temperatures will rise dramatically. To see this at work, take readings on a properly-functioning system at low fan and compare them to what you get at high fan. Then, partially restrict the inlet heater hose with a clamp and repeat the test.
This works the same with refrigerant, which explains why restricting the air flow through the evaporator core results in icing. Without air flow, the temperature drops below 32 degrees F. and the core becomes a miniature glacier.
Applications Galore
So many apps, so little space! What we’ve used it for most, and what seems perfectly obvious, is A/C work. Picture the scenario: On the first 90-deg. F. day, you get a car that’s blowing warm air. You take your IR thermometer outside and verify that in a few seconds. Yup, 80 at the ducts, hardly any refrigeration action at all. After the repair, you duplicate the measurement — now you see, say, 42 deg., and there’s nothing subjective about it. Also, your customer will be impressed by that cool “raygun.â€
| A Common MisconceptionAnon-contact infrared thermometer is not a “point and shoot†device. The accuracy of the readings you get is determined by the surface characteristics of the object under measurement. Emissivity is a quantification of how well a surface radiates or emanates IR energy, and IR energy is what these instruments use to gauge temperature. In general, the more highly polished and reflective a surface is, the lower the emissivity, and the less likely it is that you’ll be able to capture accurate data. Fortunately, the major manufacturers now offer several models with full-function processors that automatically adjust for varying emissivity. Don’t assume that readings you get are 100% accurate unless you are using the latest generation of these devices, and if in doubt take a surface reading and make the comparison so you can be sure of your data. The more you use there tools, the more uses you’ll find and the bigger and more accurate your personal database will become. Happy hunting!Emissivity Values of Various Materials
Aluminum*: 0.30Asbestos: 0.95Asphalt: 0.95Basalt: 0.70Brass*: 0.50Brick: 0.90Carbon: 0.85Ceramic: 0.95Concrete: 0.95Copper*: 0.95Dirt: 0.94Frozen food: 0.90Hot food: 0.93Glass (plate): 0.85Ice: 0.98
Iron*: 0.70Lead*: 0.50Limestone: 0.98Oil: 0.94Paint: 0.93Paper: 0.95Plastic**: 0.95Rubber: 0.95Sand: 0.90Skin: 0.98Snow: 0.90Steel*: 0.80Textiles: 0.94Water: 0.93Wood***: 0.94
*oxidized | **opaque, over 20 mils | ***natural |
To achieve max A/C performance, you may be adjusting the charge up and down by the ounce and watching the effect on duct temperature. While you could use your trusty mechanical dial thermometer, you’ll save time in this effort with that instantaneous digital reading.
Other A/C applications make sense, too. You know how a restriction in a line will sometimes cause frost to form because it’s an unintended orifice? In many cases, however, it won’t be bad enough or the conditions won’t be right for that visual indication to appear — we’ve seen compressors replaced when the real problem was choked flow. If you suspect such a performance-robbing condition, run your non-contact thermometer slowly over the line and look for a sudden change in temp.
The cooling system is another logical application. If you can get a clear shot (not so common these days), you can trace the pattern of heat rejection from the radiator as the coolant flows down to the outlet. Restrictions in individual tubes may very well disclose themselves. Peter Alexander, 1989 NAPA/ASE Technician of the Year and Illinois shop owner, says that to make the readings more obvious, he clamps off the hose until the engine gets good and hot, then releases the clamp and immediately takes his readings.
A truly helpful use is holding the beam on the thermostatic switch for the electric fan to see if it’s completing the circuit at the specified temperature. This brings to mind fan clutches — are they engaging when hot?
A related item is comparing the reading you get at the temperature sending unit to that at the gauge, which can save you from wasting time looking for a non-existent overheating problem. Also, you can arrive at a rough idea of when the thermostat opens if you monitor the temp of the water outlet or the radiator inlet as the engine warms up. Keep in mind, though, that heat conductivity and radiation will make your reading lower than the actual temperature of the coolant. How much lower? That depends on numerous factors, such as the material and thickness of the metal between the liquid and the air. You may be able to develop some standards if you experiment enough with engines that don’t exhibit any problems, but Phil Fournier, 1998 TOTY, California shop owner and esteemed MT contributor, says subtracting 10 deg. F. will put you pretty close in most cases.
Think Driveability
Are IR thermometers at all helpful in driveability/emissions/performance diagnosis? Well, some techs say using one for this is a waste of time because the readings will be inconclusive, while others are enthusiastic about the fast tip-offs they get.
Troubleshooting a miss at idle is the first thing everybody mentions. Especially with thin-wall tube-type exhaust manifolds, but also on iron to a lesser extent, you can probably find the cold runner in no time flat, and without the risks of a big-time shocks (modern ignition systems don’t only put out about a million volts, they also generate enough amperage to hurt you) or burns from yanking plug wires, or the hassle of disabling injectors. This is an especially good deal with diesels.
You know that oxygen sensors have to reach about 600 deg. F. before they start to produce voltage, right? That’s why modern specimens are electrically heated. So, if a car seems to be taking a long time to go into closed loop, or keeps falling out of that mode, you could focus on the HEGO immediately after starting the engine cold and see how fast its temperature rises compared to that of the manifold it’s screwed into — maybe the heating element or its circuit is faulty.
The coolant temperature sensor is a related application. If it’s coated with fluffy deposits, it may take an inordinate amount of time to produce accurate regulation of reference voltage, if it ever does.
EGR is a definite possibility. At speeds above idle, the valve should run hotter than the manifold it’s bolted to. No? Then look for clogged passages or lack of activation due to a bad diaphragm or a control malfunction.
In older wet throttle plate engines (in other words, TBI or carb), intake air temperature is critical. If the TAC (Thermostatic Air Cleaner) isn’t doing its job of heating those big chunks of atmosphere, hesitation is probable. First, make sure the duct is in place, then you can use you-know-what to see when that little vacuum bleed valve is causing the snorkel to open and close. Ancient history, we know, but we still get “mature†vehicles in the shop with some frequency.

… you can tell a lot about how an alternator/generator is doing by comparing its temperature to known-good specimens.
If you’re not completely confident in what OBD II is telling you about the health of that catalytic converter, you can use your IR thermometer to look for a temperature rise inlet to outlet, which will tell you that the oxidation section is working.
Nobody seems to mention the electrical system, but we can see some possibilities. For example, an energized relay will be warmer than it is when de-energized. In slow-crank situations, you might find the site of excessive resistance by checking the connections, cables, or the starter motor itself. Also, why has that alternator worked itself to death? If it’s running hotter than other specimens you’ve checked, think about a big draw or bad bearings.
Transmission Mission, Brakes and Tires
The biggest killer of automatic transmissions is overheating. So, is that trans cooler inside the radiator doing its job? Unfortunately, it’s pretty hard to figure out what’s actually happening on the road by comparing ATF line temps in your garage. You could torque it up to increase heat, but you still won’t have a spec. All we can say is that the ATF shouldn’t run at a temperature much higher than that of the coolant.
As far as the rest of the driveline is concerned, you may be able to distinguish which CV joint is making that humming noise by comparing temperatures, just as you might have more luck zeroing-in on a faulty wheel bearing if you were privy to heat readings for each.
If you’ve got a brake pull problem, or premature pad wear on one side, you might say you don’t need any diagnostic aids to recognize the trouble, but an IR thermometer may speed the process and help you avoid jumping to unfortunate conclusions. In fact, this is a use just about everybody mentions. If the caliper/rotor on the side opposite to that of the pull is cooler than its mate, a sluggish piston is likely. If the side with excessive wear is hotter than the other, you should again suspect the piston, although it’s possible that a restricted hose is allowing pressure into the cylinder, but won’t let it back out at the wimpy urgings of that piston seal.
Tire tread temperature measurement is useful mostly on the racetrack (make sure you keep the tool close, no more than five inches from the tire). Sure, too much toe-in on a regular car can heat up that rubber, but will affect both sides, and what would you compare your readings to, anyway? Besides, you’ll have visual indications. Low tires can be detected by — what a concept! — pressure readings. On the other hand, if the problem is internal disintegration that results in squirming belts and rubber, you may indeed find a significant temperature difference between that tire and its mate on the other side.
Housekeeping
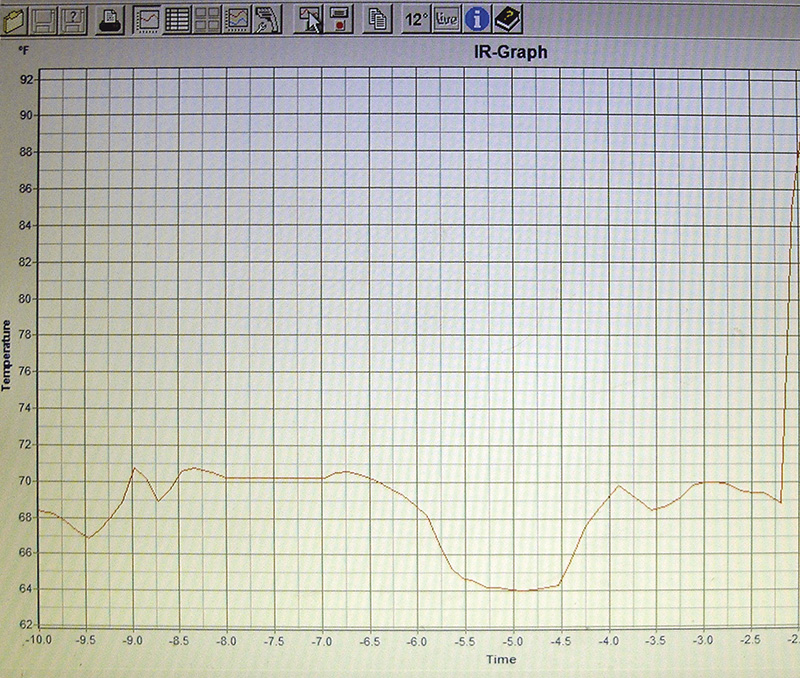
Some premium non-contact IR thermometers give you the capability of easily graphing readings on your PC. Plenty of diagnostic possibilities here.
IR thermometers aren’t just for cars, you know! How about around the shop? Measure power panel circuit breaker temperatures and record your normal readings inside the panel door, lighting ballast temperatures, HVAC duct temperatures, water heaters, tube heat or shop furnace temperatures, waste oil furnace drum temperatures (record readings when freshly cleaned to monitor ash buildup), furnace, flue and stack temperatures, monitor window, door and building insulation by checking for leaks. I’ve even used mine to find out if an employee really had a fever by shooting into his ear!

There are plenty of other uses for an IR thermometer in our business. For instance, how’s that circuit doing? Maybe you need to rewire.
Finally, some premium IR thermometers include a cable you can connect to the RS-232 terminal or a USB port of your PC, and software that lets you graph readings. This is interesting to use and can give you a picture that may trigger a solution to a knotty problem.
If you don’t own one of these little jewels, you need to get one.
Special thanks for Doug Goodwin at Extech for his assistance with this feature.
Â
Â







0 Comments